A brand-new drug, donanemab, is being hailed as a turning point in the battle versus Alzheimer’s, after a worldwide trial verifies it slows cognitive decrease.
The antibody medication assists in the early phases of the illness by clearing a protein that develops in the brains of individuals with this kind of dementia.
Although not a treatment, charities state the lead to the journal JAMA mark a brand-new age where Alzheimer’s can be dealt with.
The UK’s drugs guard dog has actually begun evaluating it for possible NHS usage.
The drug operates in Alzheimer’s illness, not in other kinds of dementia, such as vascular dementia.
In the trials, it appears to have actually slowed the rate of the illness by about a 3rd, permitting individuals to keep more of their everyday lives and jobs, such as making meals and delighting in a pastime.
Mike Colley, who is 80, is among just a few lots clients in the UK to participate in the international trial. He and his household spoke specifically with the BBC.
Mike gets an infusion every month at a center in London and states he is “among the luckiest individuals you’ll ever fulfill”.

Mike Colley (L) with his kid Mark
Mike and his household discovered he was having issues with memory and decision-making, not long prior to he began on the trial.
His kid, Mark, stated it was really tough to see at the start: “Seeing him battle with processing details and resolving issues was really hard. However I believe the decrease is reaching a plateau now.”
Mike, who is from Kent, stated: “I feel more positive every day.”
Donanemab, made by Eli Lilly, operates in the very same method as lecanemab– established by business Eisai and Biogen– which produced headings worldwide when it was shown to slow the illness.
Although very appealing, these drugs are not safe treatments.
Brain swelling was a typical side-effect in as much as a 3rd of clients in the donanemab trial. For a lot of, this fixed without triggering signs. Nevertheless, 2 volunteers, and potentially a 3rd, passed away as an outcome of unsafe swelling in the brain.
Another antibody Alzheimer’s drug, called aducanumab, was just recently declined by European regulators over security issues and an absence of proof that it worked enough for clients.
What is dementia and what can be done about it?
In the donanemab trial, scientists analyzed 1,736 individuals aged 60 to 85 with early-stage Alzheimer’s.
Half of them got a month-to-month infusion of the treatment and the other half were provided a dummy drug, likewise called a placebo, over 18 months.
- The drug appears to have a significant advantage, a minimum of for some clients
- Those who had earlier illness and less brain amyloid at standard obtained higher advantage, in regards to clearance seen on brain scans
- Those provided the drug likewise kept more of their everyday lives such as having the ability to go over existing occasions, respond to the phone or pursue pastimes
- The rate of the illness, evaluated by what individuals might still do everyday, was slowed by about 20-30% general– and by 30-40% in a set of clients who scientists believed most likely to react
- There were substantial side-effects and clients will require to be knowledgeable about threats of treatment
- Half of clients on donanemab had the ability to stop the treatment after a year, due to the fact that it had actually cleared enough brain deposits
Amyloid is simply one part of the intricate image of Alzheimer’s, and it is uncertain if the treatment will continue to make more distinction over a longer duration, specialists warn.
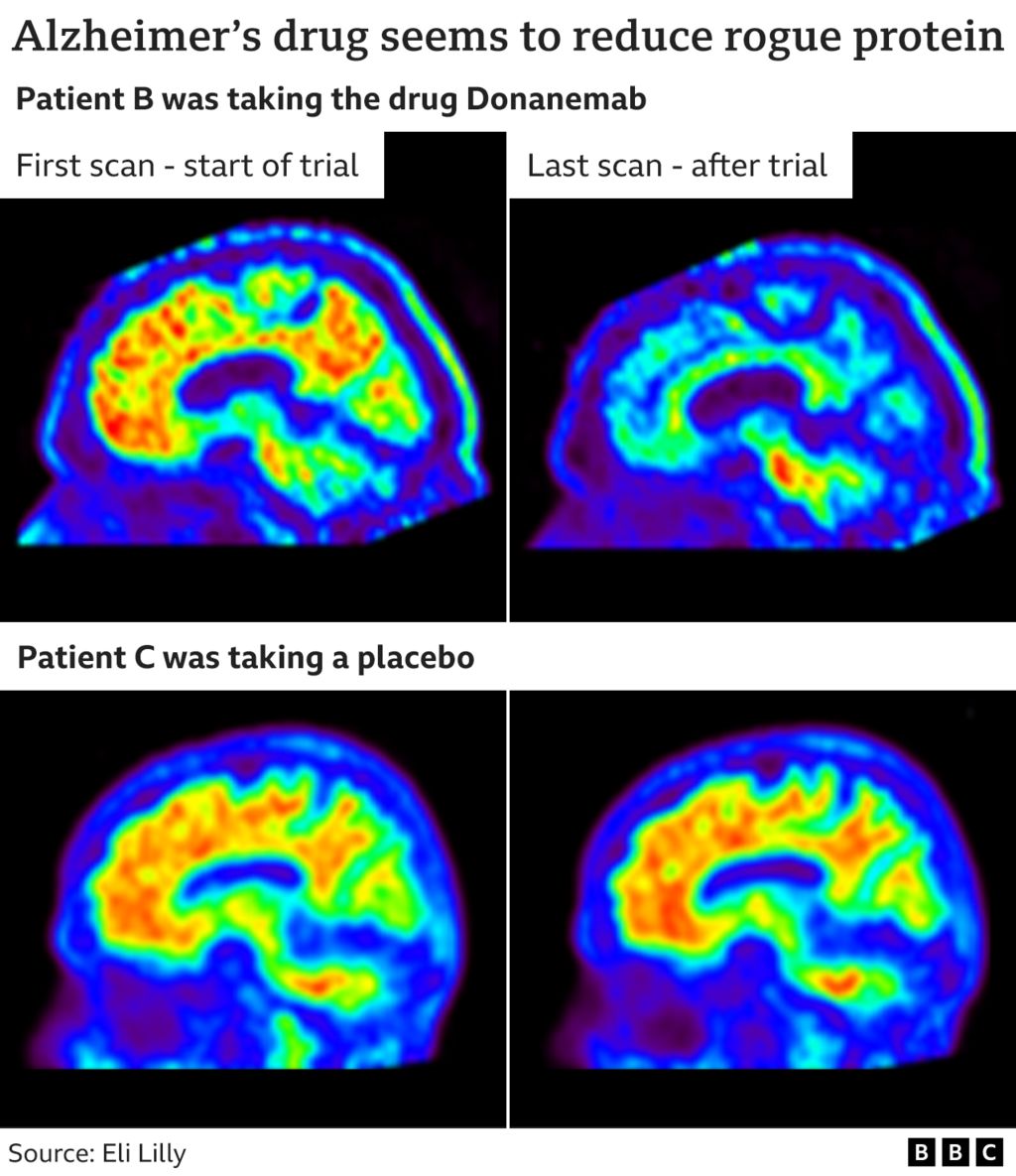
Prior to and after scans demonstrate how the drug cleared deposits (seen in green, yellow and red) from the brain
The drug’s results might be modest, however the outcomes offer more verification that getting rid of amyloid from the brain might alter the course of Alzheimer’s, and assist individuals impacted by this ravaging illness if they’re dealt with at the correct time, they state.
Prof Giles Hardingham from the UK Dementia Research study Institute stated: “It is fantastic to see these outcomes released completely today.
” We have waited a long period of time for Alzheimer’s treatments, so it’s truly motivating to see concrete development continuing to collect rate in the field.”
Dr Susan Kohlhaas, from Alzheimer’s Research study UK, stated: “Today’s statement marks another turning point.
” Thanks to years of research study, the outlook for dementia and its effect on individuals and society is lastly altering, and we’re getting in a brand-new age where Alzheimer’s illness might end up being treatable.”
Speaking With BBC Radio 4’s PM program, previous Prime Minister David Cameron stated resources need to be put towards more research study into what he called a “statin for the brain”.
” We desire a tablet that individuals who have the accumulation of these proteins in the brain can take every day or weekly in order to clear those proteins out of the brain and for that reason minimize your opportunities of getting an illness that triggers dementia,” he stated.
Asked if the federal government were prepared to invest where required to present brand-new treatments, Mr Cameron stated there was a genuine reward to do so: “We’re a nation of sixty million individuals, with a million individuals with dementia, a number of them in really pricey property care settings therefore there is a great deal of cost savings to be had from successfully dealing with individuals … I’m enthusiastic that our system can provide.”
Lecanemab expenses around $27,500 (⤠21,000) in the United States, where it is certified.
It is unclear just how much donanemab might cost and the length of time it may require to get approval in the UK, however Alzheimer’s specialists stated having 2 drugs would assist promote competitors on rate.
The UK’s drug’s guard dog NICE states it has actually currently begun deal with its appraisal of donanemab for dealing with moderate cognitive problems or moderate dementia triggered by Alzheimer’s illness.
” Our goal is to produce suggestions on its usage in the NHS as close as possible to it getting its UK licence,” stated a representative.
Mike Colley turned 80 in April. At his birthday celebration, he amazed his household by singing My Method front of 40 visitors.
He informed BBC News: “That’s the self-confidence I have now. I ‘d never ever have actually done that even 12 months earlier.”
His kid Mark included: “I never ever believed I would see my papa so complete of life once again. It was an extraordinary minute.”
Dr Emer MacSweeney, specialist neuroradiologist and medical director at Re: Cognition Health, led the trials of donanemab in the UK.
She stated: “This is truly substantial and among the most significant advancements.”
The Alzheimer’s Society stated: “This is genuinely a turning point in the battle versus Alzheimer’s and science is showing that it is possible to decrease the illness.”
Around 720,000 individuals in the UK may possibly gain from these emerging brand-new Alzheimer’s illness treatments if they’re authorized for usage, however the Alzheimer’s Society stated the NHS is “merely not prepared to provide them”.
Kate Lee, CEO for the charity, stated: “Timely, precise medical diagnosis is crucial, and presently just 2% of individuals in England and Wales get their medical diagnosis through the expert examinations required to be qualified for these treatments.
” Together with this, these emerging Alzheimer’s illness drugs need routine infusions and tracking, and the NHS is not yet geared up to do this at scale.”